Medical Device and Pharmaceutical Recalls: Is Your Organization 100% Protected?
It May Be Time to Evaluate How Recalls are Being Identified in Your Healthcare Facility
february 2022
By Jody Randall MSN, RN, CIC, HACP-CMS, HACP-PE
CEO and Founder
Pharmaceutical and medical device recalls are nothing new in the healthcare industry. It is important to understand that recalls may happen for a variety of reasons. It is also important to understand that the level of risk associated with the recalled medication or equipment is categorized based on the level or risk associated with the product. This is to help communicate from how minor to how dangerous the product is if used in a conventional manner. Although the Food and Drug Administration (FDA) enforces standards for recalling vaccines, medical devices, and pharmaceuticals; the responsibility is that of the manufacturer of these types of devices to report information about quality concerns of the products that have identified. The FDA does however have the authority to issue a mandatory recall based upon the severity of risk associated with any of these products.
Once a manufacturer has determined that a medication or device needs to be recalled for review, modification or removed from the market completely, the manufacturer should then immediately notify not only the FDA but all clients who may have purchased the item(s) in question. Notification letters should include a priority status. If a device or medication could potentially cause serious harm or even death, it would be classified as a Category 1 risk by the FDA and marked urgent by the sender of the notification. For moderate risk, items or medications would be labeled as a Category 2 risk which can still lead to health risks that do not result in irreversible harm or fatality. Category 3 risks are considered to be minor. These may be associated with a mere design flaw but in no way are expected to result in adverse outcomes if they do reach the patient.
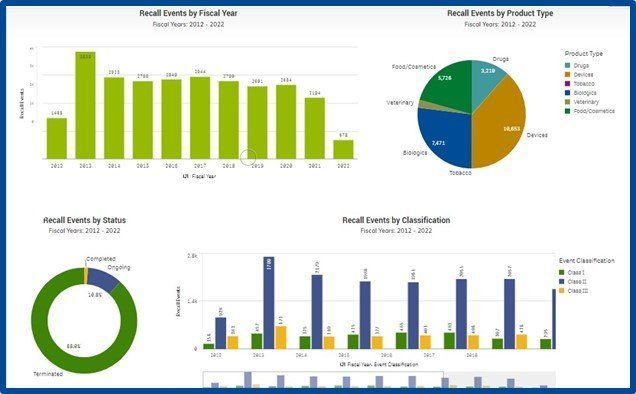
It may be a good time to familiarize your pharmacists and supply chain team members with the FDA’s recall websites listed under resources below. The list includes an eye-opening number of medical device and pharmaceutical recalls in addition to non-medical products recalled. It may also be a good time to conduct a risk assessment for your organization to determine how recalls are managed on a day-to-day basis.
There are a variety of sophisticated informatics systems that help healthcare organizations communicate this vital information to key stakeholders upon receipt of the information. In the consulting world, we often encounter a variety of issues resulting in vital information not being communicated properly or effectively. Inefficient or broken lines of communication and/or not updating current contact information for essential personnel can result in serious risk to patients. Inefficient or ineffective communication of recalls can easily open organizations up to financial and legal consequences.
We know how overwhelming the constant influx of information is in the healthcare world right now. We are always here to help! Let us help your organization get back on track today.
From all of us here at HCE, we are extremely grateful for all you do, and we hope you all are staying safe out there!
Please join us by clicking on any of the icons below to leave a comment or for more informati
on and updates:





